Metal-Organic Frameworks as Porous Hosts for Photoswitchable Dye Molecules
Synthesis of Switch@MOF composite materials using different photochromic dyes, which are embedded into Metal-Organic Frameworks, to investigate solid-state switching and solvatochromic effects.
Main Publication:
Solution-like Behavior of Photoswitchable Spiropyrans Embedded in Metal–Organic Frameworks
H. Schwartz, S. Olthof, D. Schaniel, K. Meerholz, U. Ruschewitz, Inorg. Chem. 2017, 56, 13100−13110.
Usually, photoactive molecules such as azobenzenes and spiropyrans only show photochromic behavior in solution, since the light induced switching processes require a certain degree of sterical freedom. In recent years we embedded azobenzenes and fluorinated derivatives as well as spiropyrans as non-covalently attached guest molecule into different MOF host matrices.
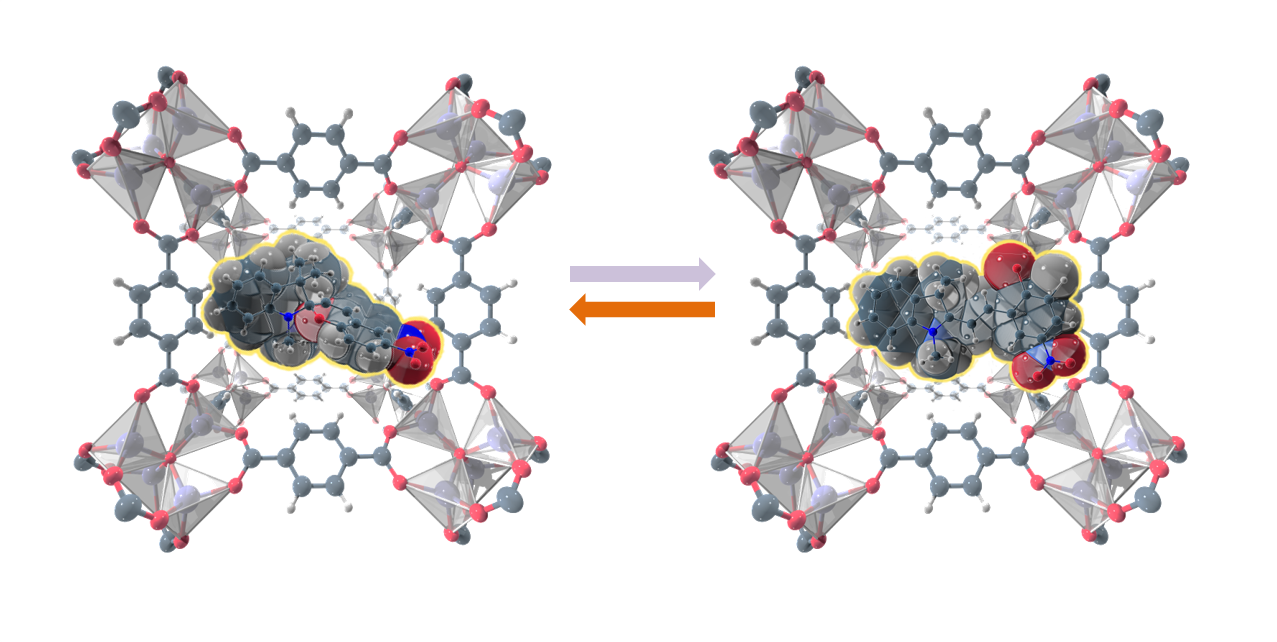
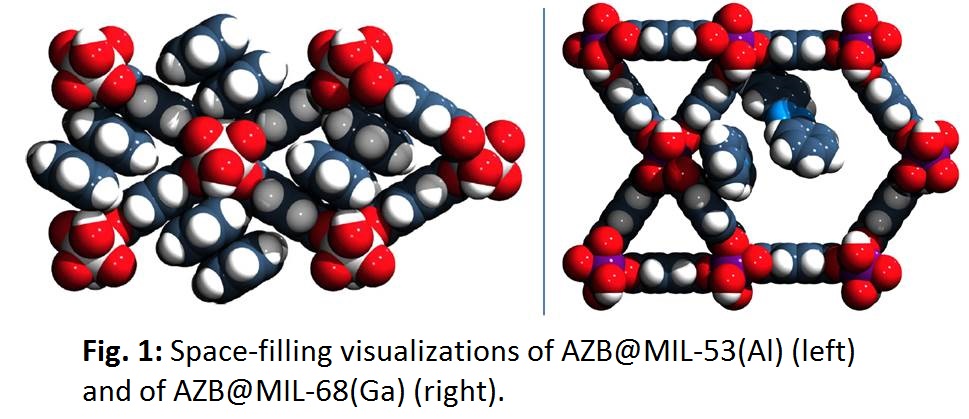
Interestingly, switching was found to be more efficient for some of the azobenzene compounds, whereas for MIL-53 as host matrix light triggered changes were not observed. By means of high resolution synchrotron powder diffraction the crystal structure of this composite material was determined (see Figure 2) pointing to the reason of inhibited photoswitching: because of the dense packing inside the MOF pore the isomerization of azobenzene was sterically hindered. This is the first insight into structure-property relationships of a guest@MOF system. We showed that consequently, size and shape of the host pore as well as the orientation and the amount of the embedded guest molecules within the pores have a significant impact on the resulting optical properties of the photoswitch@MOF systems.
For spiropyrans, solid-state photochromism was found to occur as well when embedded into different MOF host materials. Additionally, the incorporated spiropyran also showed solvatochromic response, pointing to different chemical environments inside the used MOF host materials. Consequently, MOFs can be understood as “solid solvents” for these dye molecules (see Figure 3).
Further Publications
[1] S. Garg, H. Schwartz, M. Kozlowska, A. Bruno Kanj, K. Müller, W. Wenzel, U. Ruschewitz, L. Heinke.
Angew. Chem. Int. Ed. 10.1002/anie.201811458.
[2] H. A. Schwartz, U. Ruschewitz, L. Heinke, Photochem. Photobiol. Sci. 2018, 17, 864-873.
[3] D. Hermann, H. A. Schwartz, U. Ruschewitz, ChemistrySelect 2017, 2, 11846-11852.
[4] K. Müller, J. Wadhwa, J. S. Malhi, L. Schöttner, A. Welle, H. Schwartz, D. Hermann, U. Ruschewitz, L. Heinke, Chem. Commun. 2017, 53, 8070-8073.
[5] D. Hermann, H. Emerich, R. Lepski, D. Schaniel, U. Ruschewitz, Inorg. Chem. 2013, 52, 2744-2749.